Iptacopan delivers sustained QoL gains and symptom control in PNH: 48-week findings from the APPLY-PNH and APPOINT-PNH trials
3 Apr 2025
Share
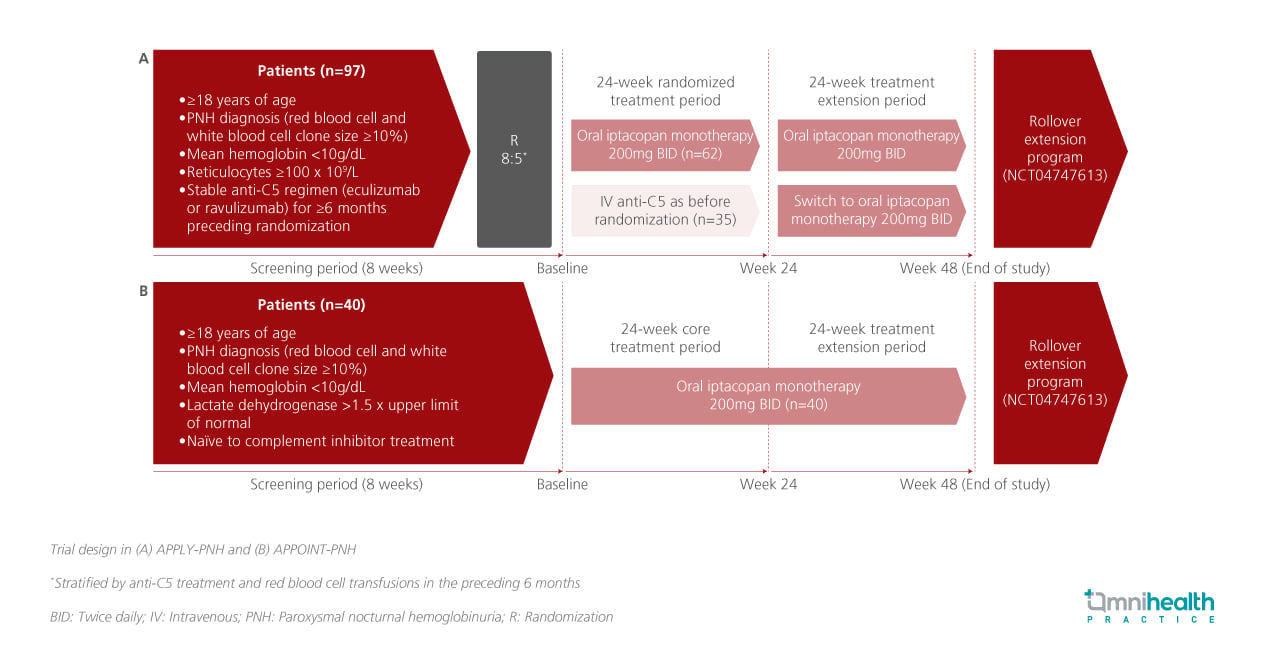
STUDY DESIGN
Persistent hemolysis and anemia in paroxysmal nocturnal hemoglobinuria (PNH) can significantly impair health-related quality of life (HRQoL) and lead to debilitating symptoms, with fatigue recognized as the most disabling.1 Despite advancements, a need remains to address these challenges effectively.1 Iptacopan is an oral proximal complement inhibitor targeting factor B in the alternative pathway approved to treat adults with PNH.1 As part of the phase 3 APPLY-PNH and APPOINT-PNH trials, data on patient-reported HRQoL outcomes and investigator-assessed signs and symptoms of PNH over 48 weeks of iptacopan monotherapy were analyzed.1
APPLY-PNH was an open-label, randomized, multicenter, phase 3 trial conducted in adult patients with PNH and mean hemoglobin (Hb) 1.5x the upper limit of normal, who received iptacopan (200mg twice daily, n=40) for 24 weeks.1 Patients in both trials, including those initially on anti-C5 therapy in APPLY-PNH, were allowed to continue iptacopan monotherapy for an additional 24 weeks, completing a total of 48 weeks of treatment.1
The exploratory endpoints presented included changes in patient-reported HRQoL using the European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire Core 30 (EORTC QLQ-C30) questionnaire, and investigator-assessed PNH signs and symptoms (e.g. hemoglobinuria, fatigue, dyspnea, chest/abdominal pain, and dysphagia) recorded at each visit.1
FINDINGS
| Exploratory analysis |
| • The exploratory endpoints included changes in patient-reported HRQoL from the EORTC QLQ-C30 questionnaire, and investigator-assessed PNH signs and symptoms (e.g., hemoglobinuria, fatigue, dyspnea, chest/abdominal pain, and dysphagia) recorded at each visit1 |
| Patient-reported HRQoL (APPLY-PNH): |
| • At week 48, improvements in all functional domains of EORTC QLQ-C30 (cognitive +9.5 [15.8], emotional +11.7 [23.6], physical +14.7 [16.4], role +14.4 [23.8], social +13.2 [30.3]), global health status (+16.3 [18.0]), dyspnea (-28.8 [29.5]), and fatigue (-18.2 [23.5]) were observed1 |
| Patient-reported HRQoL (APPOINT-PNH): |
| • At week 48, improvements in all functional domains of EORTC QLQ-C30 (cognitive +2.4 [22.8], emotional +8.3 [23.4], physical +16.2 [17.5], role +16.9 [22.9], social +13.2 [28.1]), global health status (+22.0 [21.3]), dyspnea (-17.5 [28.6]), and fatigue (-22.2 [25.0]) were observed1 |
| Investigator-assessed PNH symptoms (APPLY-PNH): |
| • At baseline, 62.9% of iptacopan arm and 68.6% of anti-C5 arm had ≥1 PNH symptom. The most common symptoms reported were weakness/tiredness, dyspnea1 |
| At week 48, 25.8% of iptacopan arm, and 26.5% of anti-C5-to-iptacopan arm had ≥1 PNH symptom1 |
| • At week 48, the proportion of patients not experiencing weakness/tiredness increased from 48.4% at baseline to 75.8% in the iptacopan arm, while it increased from 31.4% at baseline to 82.4% in anti-C5-to-iptacopan arm at week 24 through week 481 |
| • Similarly, proportion of patients free of dyspnea symptom increased from 71.0% of iptacopan arm at baseline, to 87.1% at week 48 while it increased from 62.9% to 85.3% of anti-C5-to-iptacopan arm at week 24 through week 481 |
| Investigator-assessed PNH symptoms (APPOINT-PNH): |
| • At baseline, 97.5% had ≥1 PNH symptom. The most common symptom reported was reddish or cola-colored urine, especially in the morning, or hemoglobinuria1 |
| • At week 48, 27.5% of patients had ≥1 PNH symptom, while 95.0% of patients were free of reddish or cola-colored urine, especially in the morning, or hemoglobulinuria1 |
| • Improvements in the other PNH symptoms were also observed over 48 weeks1 |
“These results provide further evidence of the long-term positive impact of iptacopan on HRQoL and common signs and symptoms of PNH”
Presented by
Professor Antonio M Risitano
AORN Moscati,
Avellino, Italy
References
- Risitano AM, et al. Oral iptacopan monotherapy leads to long-term improvements in patient-reported health-related quality of life (HRQoL) and investigator-assessed signs and symptoms of paroxysmal nocturnal hemoglobinuria (PNH): 48-Week results from the Phase III APPLY-PNH and APPOINT-PNH trials. Presented at the 66th American Society of Hematology (ASH) Annual Meeting 2024. December 7-10, 2024.