Nivolumab + chemotherapy improves long-term survival outcomes in resectable NSCLC: Final OS analysis from the CheckMate 816 trial
7 Sep 2025
Share
STUDY DESIGN
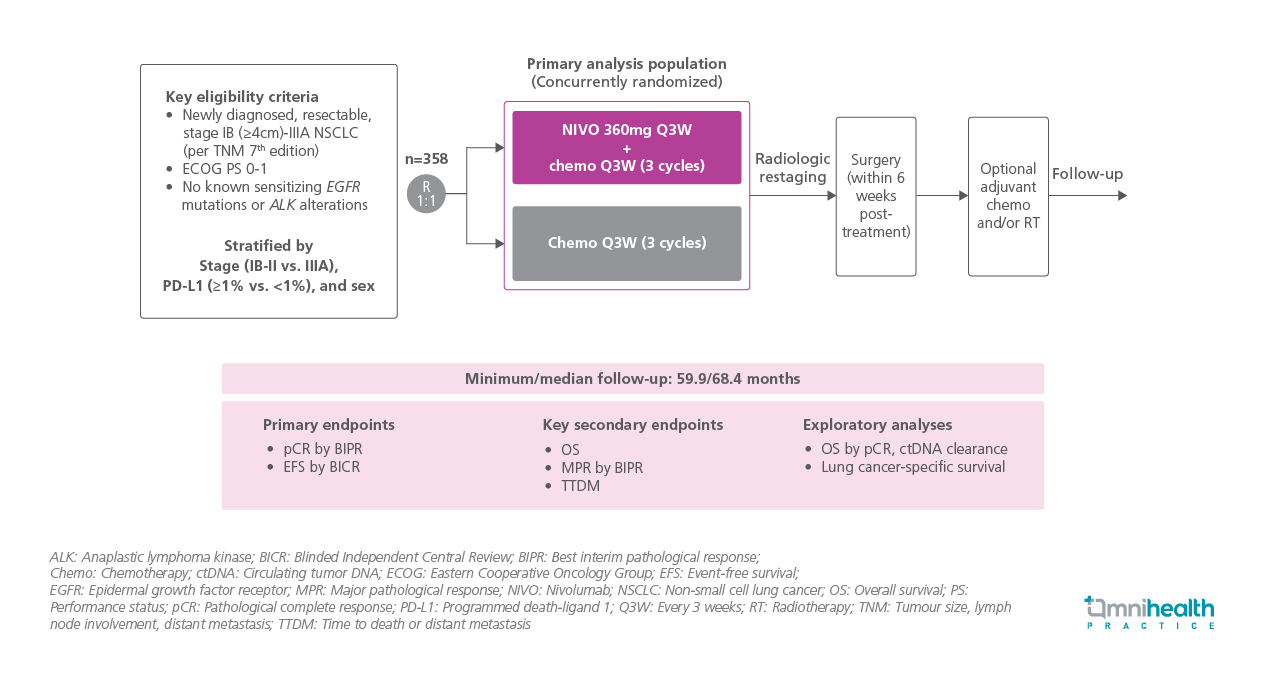
Nivolumab + chemotherapy is an established standard-of-care neoadjuvant treatment for eligible patients with resectable non-small cell lung cancer (NSCLC).1 It is the sole neoadjuvant-only chemoimmunotherapy regimen approved in the United States, the European Union, and some other countries.1 In the phase 3 CheckMate 816 study, nivolumab + chemotherapy demonstrated statistically significant and clinically meaningful improvements in event-free survival (EFS) and pathological complete response (pCR) compared with chemotherapy alone in patients with resectable NSCLC.1 In this preplanned final analysis of overall survival (OS) from the trial, the efficacy and safety of neoadjuvant nivolumab + chemotherapy were evaluated over a minimum follow-up of 5 years.1
The trial enrolled 358 participants with newly diagnosed, resectable stage IB (≥4cm)-IIIA NSCLC per tumor, node, metastasis (TNM) 7th edition, Eastern Cooperative Oncology Group Performance Status (ECOG PS) ≤1, and no known EGFR mutations or ALK alterations.1 Patients were stratified by disease stage, programmed death-ligand 1 (PD-L1) tumor proportion score (TPS), and sex.1
Participants were randomized 1:1 to receive neoadjuvant nivolumab 360mg + chemotherapy (n=179) or chemotherapy alone (n=179), administered every 3 weeks for 3 cycles prior to surgery.1 Surgical resection was planned to occur within 6 weeks after completion of neoadjuvant therapy.1 Postoperative adjuvant chemotherapy, radiotherapy, or both were permitted.1
The dual primary endpoints were pCR and EFS.1 The key secondary endpoint presented was overall survival (OS).1 Exploratory analyses included OS stratified by circulating tumor DNA (ctDNA) clearance and pCR status, along with lung cancer-specific survival.1
FINDINGS
| Primary endpoints: |
|
|
|
|
|
| Key secondary endpoint: |
|
|
|
|
|
| Exploratory analysis: |
|
|
|
| Safety : |
|
|
|
|
|
References
- Forde PM, et al. Nivolumab plus chemotherapy in resectable non-small cell lung cancer: Final results from the phase 3 CheckMate 816 trial. Presented at the 2025 American Society of Clinical Oncology (ASCO) Annual Meeting; May 30-June 3, 2025.