Seladelpar reduces cholestatic and liver injury markers in patients with PBC: Results from the RESPONSE trial
10 Apr 2024
Share
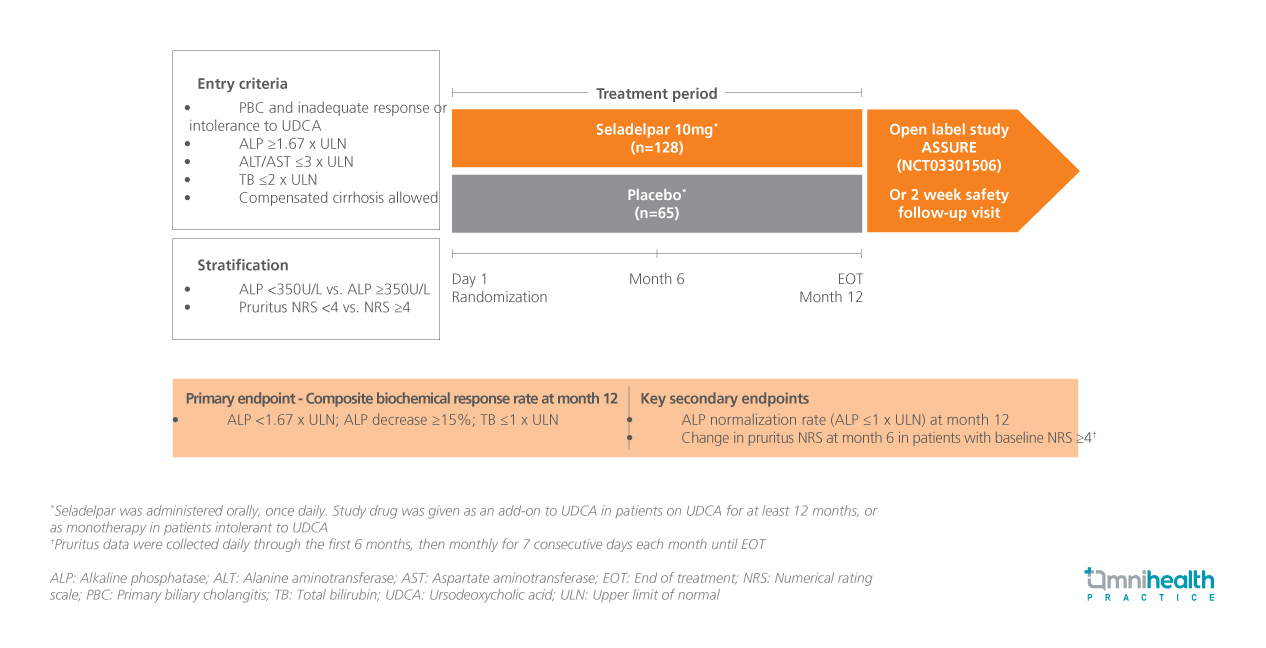
STUDY DESIGN
Primary biliary cholangitis (PBC) is a chronic autoimmune liver disease that primarily affects women and is characterized by the progressive destruction of bile ducts.1 This condition can lead to liver injury, fibrosis, and ultimately cirrhosis.1 To address the unmet needs of patients inadequately responding to ursodeoxycholic acid (UDCA), seladelpar, a selective peroxisome proliferator-activated receptor delta (PPARδ) agonist, has emerged as a promising treatment option.1 The phase 3 RESPONSE trial was designed to evaluate the efficacy and safety of seladelpar in patients with PBC.1
The RESPONSE trial was a placebo-controlled study involving 193 patients with PBC who had either an inadequate response to or a history of intolerance to UDCA.1 Patients were randomly assigned in a 2:1 ratio to receive either 10mg of the seladelpar (n=128) or placebo (n=65) daily for 12 months.1 The mean age of these patients was 59.5 years.1 All patients in the treatment group were female, while 78% of the placebo group were female.1
In this presentation, efficacy outcomes in the subset of patients with PBC with (n=17) or without cirrhosis (n=166) were reported and included changes from baseline in alkaline phosphatase (ALP), gamma-glutamyl transferase (GGT), alanine aminotransferase (ALT), and total bilirubin (TB) levels.1
FINDINGS
| Efficacy endpoints: |
|
|
|
|
|
|
|
| Safety: |
|
|
|
|
|
|
|
“In patients with PBC and cirrhosis, seladelpar decreased cholestatic and liver injury markers compared with placebo, similar to effects seen in patients without cirrhosis in the RESPONSE trial”
Dr. Alejandra Villamil
The Liver Autoimmunity Unit,
Hospital Italiano de Buenos Aires, Buenos Aires,
Argentina
References
- Villamil A, et al. Efficacy and Safety of Seladelpar in Patients with Primary Biliary Cholangitis and Compensated Cirrhosis in the Phase 3 Placebo-Controlled RESPONSE Trial: 3-Year Results. Presented at the 66th American Association for the Study of Liver Diseases (AASLD) Annual Meeting 2024; November 15-19, 2024.