Tag : ATOPIC DERMATITIS

Abrocitinib demonstrates rapid and sustained effectiveness in treating atopic dermatitis in Chinese patients, based on AHEAD study interim findings. Over 52 weeks, significant improvements in pruritus, skin lesions, QL, and reduced economic burden highlight its value as a long-term treatment for moderate to severe AD.

A global epidemiological study found that those with atopic dermatitis (AD) had a higher prevalence of SI compared to those without AD, regardless of onset age. The study also identified several demographic and clinical predictors for SI, emphasizing the importance of recognizing and managing suicide risk in patients with AD.

Hidradenitis suppurativa (HS) is a chronic inflammatory skin condition that significantly impacts patients' quality of life (QoL). Individuals with HS often experience comorbidities, including psychiatric conditions such as depression and anxiety, with recent findings indicating they are about twice as likely to develop these disorders compared to those without HS. Secukinumab has shown sustained efficacy in moderate to severe HS in the SUNSHINE and SUNRISE phase 3 trials, improving QoL while maintaining a favorable safety profile over one year.

Baricitinib is a selective Janus kinase inhibitor approved in over 70 countries for treating moderate-to-severe atopic dermatitis (AD) in adults. In the European Union, Saudi Arabia, and Japan, it is also approved for children and adolescents aged 2 years and older with moderate-to-severe AD who are eligible for systemic therapy. Previous integrated analyses showed that baricitinib maintained a similar safety profile with no new safety signals, and the incidence of adverse events of special interest during long-term follow-up remained low.

Dysregulation of the human microbiome has been a common clinical feature observed in patients with atopic dermatitis (AD) and food allergy (FA). Since diet can lead to fluctuations in the microbiome, dietary modifications may serve as modulators for skin microbial composition.1 In mouse models, diets low in fatty acids have been associated with impairment in epithelial barrier functions. In addition, patients with both AD and FA have more severe epithelial barrier function impairment than those with AD only, further suggesting that dietary fat is essential for skin barrier function. Nevertheless, studies that investigated the efficacy of dietary modifications in the management of AD has been limited due to inconsistent outcomes and an incomplete understanding of the complex interactions behind the skin and gut microbiomes. During the 2024 AAAAI Annual Meeting, Dr. John Fyolek from Northwestern University Feinberg School of Medicine, the United States, shared the results of a study examining the effect of high-fat diets on skin microbiome in children with food allergies and eczema.

Early identification of high-risk infants for atopic dermatitis (AD) in the pre-disease stage can be crucial as it allows more well-timed and effective targeted intervention for AD prevention.1 During the 2024 AAAAI Annual Meeting, Dr. Elizabeth Tham from the National University of Singapore, presented the results of a sub-study within the Singapore Preconception Study of Long-Term Maternal and Child Outcomes (S-PRESTO), which aimed to identify signatures of early skin microbiome, natural moisturizing factors (NMFs) and anti-microbial peptides (AMPs) that can predict infantile AD at 6 and 18 months of life.
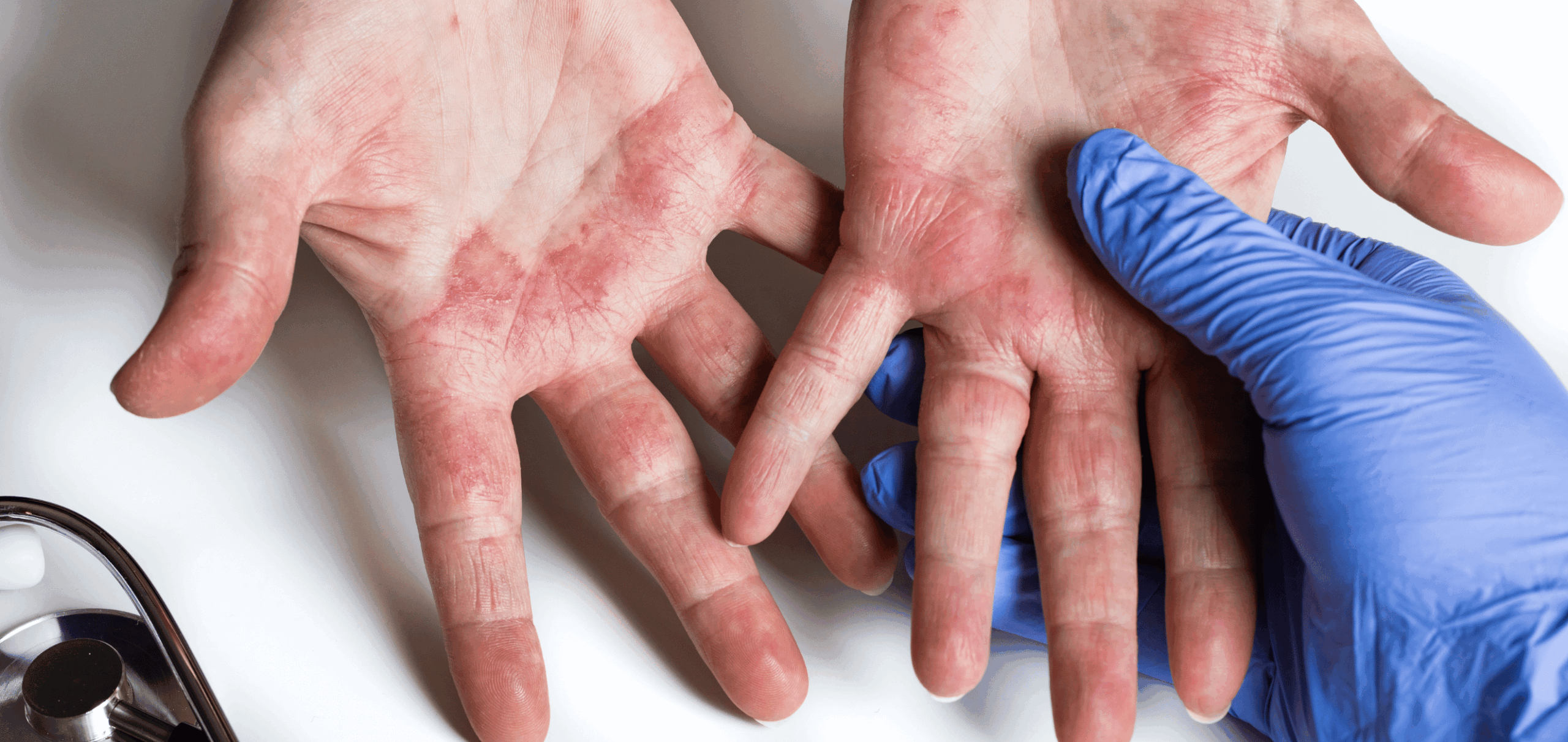
Despite being approved for the treatment of moderate-to-severe atopic dermatitis (AD), dupilumab’s efficacy in treating patients with atopic hand and foot dermatitis had not been evaluated. The LIBERTY-AD-HAFT trial demonstrated dupilumab’s efficacy and safety in treating patients with AD with moderate-to-severe hand and foot involvement. Based on the study results, the United States (US) Food and Drug Administration (FDA) updated the label of dupilumab to include AD with moderate-to-severe hand and foot involvement.

Symptoms of atopic dermatitis (AD), including dry skin, pruritus, and skin lesions, significantly impact the quality of life (QoL) of patients. At the EADV Congress 2024, results from a real-world use of a 12-week digital health program developed to help patients with AD build healthy habits around the itch-scratch cycle, were presented by Dr. Sigrídur Lára Gudmundsdóttir from Sidekick Health, Kopavogur, Iceland.

