Tag : DUPILUMAB
Chronic spontaneous urticaria (CSU) affects approximately 1% of the global population and is often challenging to manage. First-line treatment typically involves second-generation H1 antihistamines (H1-AH), administered at doses up to four times the standard recommendation, but only around half of patients achieve adequate symptom control. Until recently, treatment options for those who remained symptomatic were limited. This highlights an unmet need for additional targeted therapies. Recently, the United States (US) Food and Drug Administration (FDA) approved dupilumab, the first new targeted treatment for CSU in over a decade, for patients aged 12 and older who remain symptomatic despite antihistamine use.

Eosinophilic esophagitis (EoE) is a chronic, progressive, type 2 inflammatory disease of the esophagus. While food elimination diets (FED) are an established treatment for EoE, their impact on the efficacy of medical therapy remains unclear.

Hidradenitis suppurativa (HS) is a chronic inflammatory skin condition that significantly impacts patients' quality of life (QoL). Individuals with HS often experience comorbidities, including psychiatric conditions such as depression and anxiety, with recent findings indicating they are about twice as likely to develop these disorders compared to those without HS. Secukinumab has shown sustained efficacy in moderate to severe HS in the SUNSHINE and SUNRISE phase 3 trials, improving QoL while maintaining a favorable safety profile over one year.

Dupilumab is indicated as an add-on maintenance treatment for adult patients with inadequately controlled chronic rhinosinusitis with nasal polyps (CRSwNP). It has been shown to improve both objective and patient-reported outcomes in this population.

Earlier this year, the United States (US) Food and Drug Administration (FDA) expanded the approval of dupilumab to include the treatment of eosinophilic esophagitis (EoE) in pediatric patients aged ≥1 year weighing at least 15 kg.
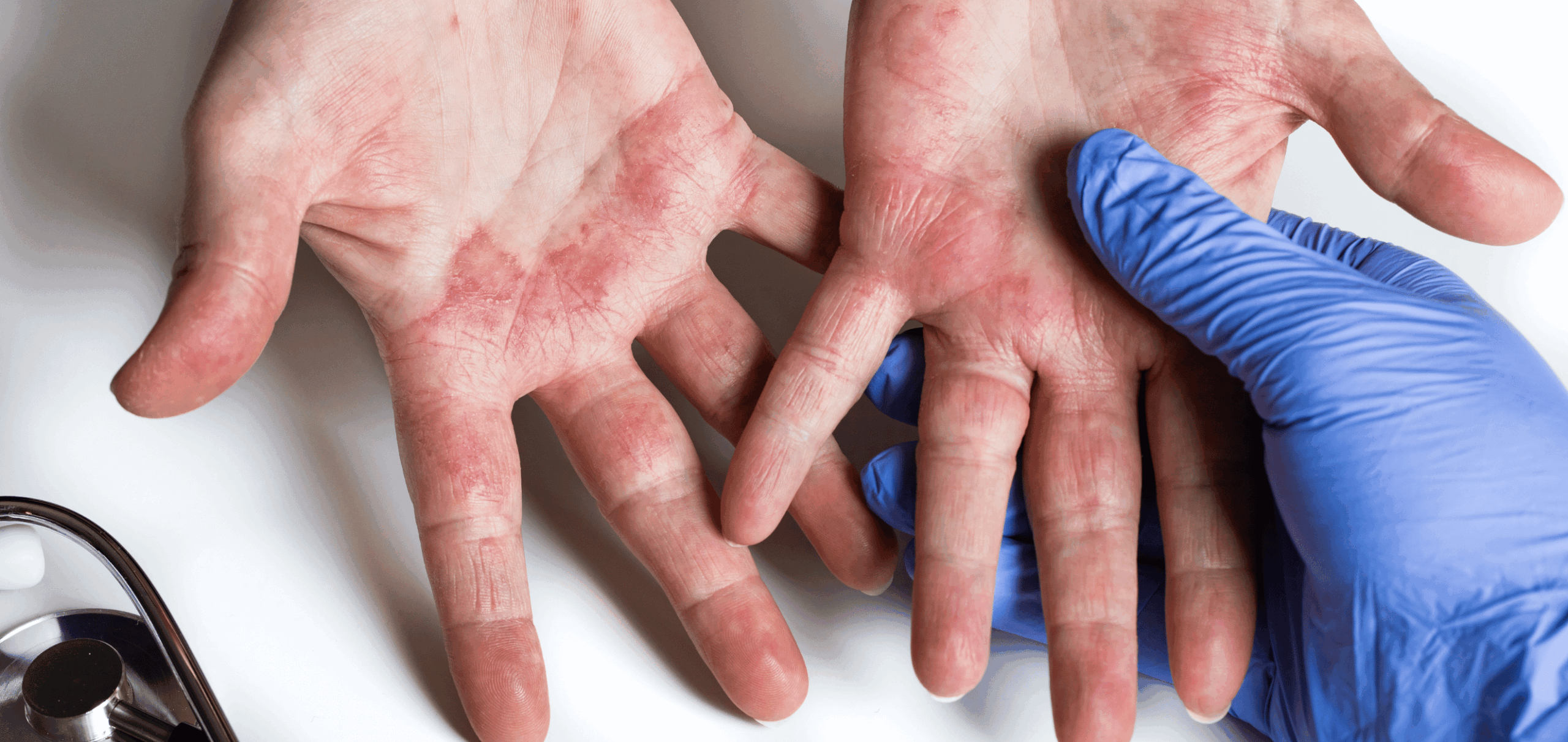
Despite being approved for the treatment of moderate-to-severe atopic dermatitis (AD), dupilumab’s efficacy in treating patients with atopic hand and foot dermatitis had not been evaluated. The LIBERTY-AD-HAFT trial demonstrated dupilumab’s efficacy and safety in treating patients with AD with moderate-to-severe hand and foot involvement. Based on the study results, the United States (US) Food and Drug Administration (FDA) updated the label of dupilumab to include AD with moderate-to-severe hand and foot involvement.
