Omalizumab shows potential against multiple food allergies: Stage 1 findings from the OUtMATCH study
30 May 2024
Share
STUDY DESIGN
Food allergy is common and can affect up to 8% of children and 10% of adults respectively, many of whom have concurrent food allergies.1 The allergy may require additional healthcare utilization, and their specific diets often leads to negative impacts on their nutrition, quality of life (QoL), and personal finances.1 Besides avoiding trigger foods and using emergency treatment when needed, there is only 1 treatment that has been approved by the Food and Drug Administration (FDA) for peanut allergy associated with burdensome therapy and high rates of adverse reactions.1 Hence, the goal of newer treatments is to target multiple allergies simultaneously, reduce reactions on accidental exposure and improve overall QoL.1 Omalizumab is a biologic approved for the treatment of allergic asthma, chronic spontaneous urticaria, and chronic rhinosinusitis with nasal polyps with a proven safety profile.1 It is potentially useful in the management of food allergy due to its non-specificity to food, intermittent dosing schedule and its ability to control comorbidities, with possible benefits on disease modification.1
The OUtMATCH study was a randomized, double-blind, placebo-controlled phase 3 study of omalizumab consisting of 3 stages.1 Patients (aged 1 to 55) allergic to peanuts and at least 2 of the following foods including milk, egg, wheat, cashew, hazelnut and walnuts, and had a body weight and total serum immunoglobulin E (IgE) level suitable for omalizumab administration were recruited into the study.1 After the initial screening process, a total of 177 patients were included in the first stage of the study.1 They were randomized (2:1) to receive omalizumab (n=118) or placebo (n=59) every 2 or 4 weeks for 16-20 weeks respectively, and had 4 oral food challenges, the results of which are presented here.1
The primary endpoint was the consumption of a single dose of ≥600mg of peanut protein without dose-limiting symptoms.1 Secondary endpoints included the consumption of ≥1,000mg of cashew protein, milk protein and egg protein respectively without dose-limiting symptoms.1 Other secondary endpoints included safety outcomes and the consumption of varying amounts (≥600mg/ ≥1,000mg/ ≥1 dose of 2,000mg/ 2 doses of 2,000mg) and types of foods (1 food/ ≥2 foods/ all 3 foods) without dose-limiting symptoms.1
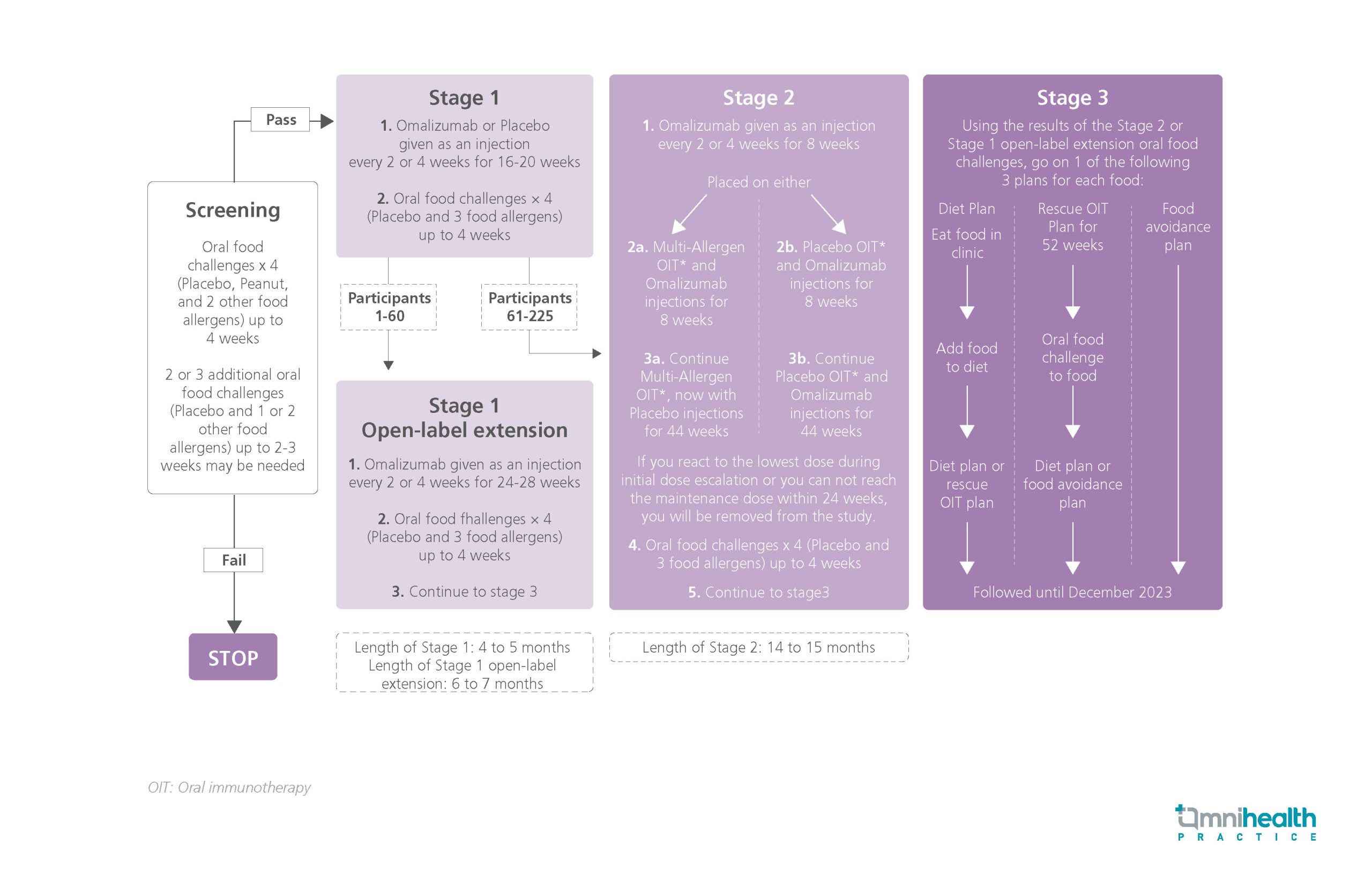
FINDINGS
| Primary endpoints: |
|
|
| Secondary endpoints: |
|
|
|
| Safety outcomes: |
|
|
“What I want is more options to treat our patients on an individual basis. Patients with simple food allergies may be doing great with food avoidance or they may be very anxious from their allergy and would benefit from some form of therapy”
Dr. Robert A. Wood
Deputy director at Institute of Clinical and Translational Research,
Johns Hopkins University,
USA
References
- Wood RA, et al. Omalizumab for the Treatment of Food Allergy: The OUtMATCH Study. Presented at the 2024 American Academy of Allergy, Asthma & Immunology (AAAAI) Annual Meeting; February 23-26, 2024.


