Fremanezumab demonstrates efficacy and safety in pediatric patients with episodic migraine: Results from the phase 3 SPACE study
1 May 2025
Share
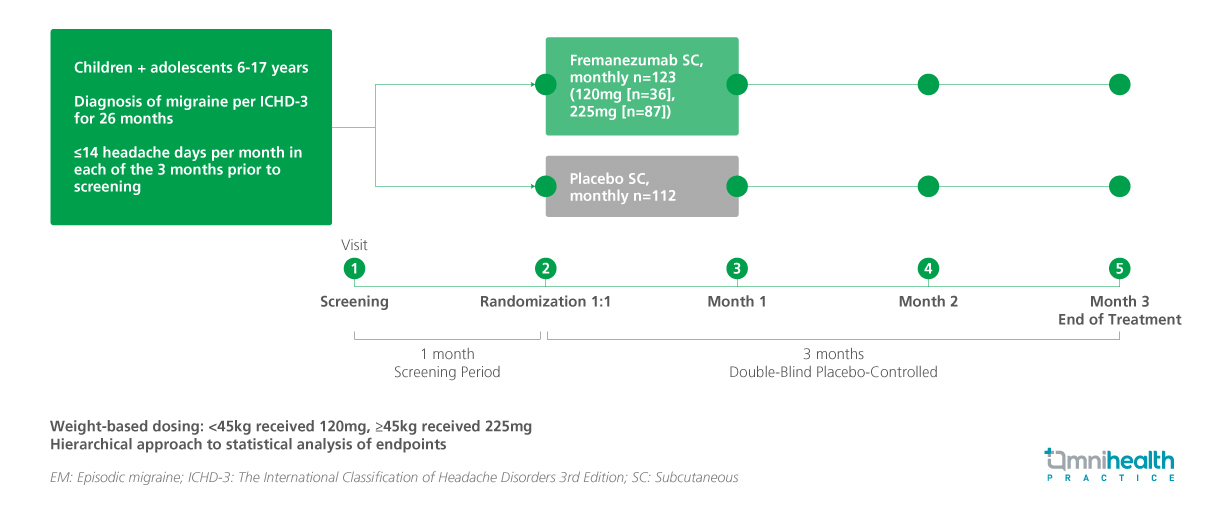
STUDY DESIGN
Approximately three-quarters of children and adolescents with migraine will continue to experience migraine into adulthood, contributing to a significant long-term disease burden in this population.1 The phase 3 SPACE trial was designed to evaluate the efficacy and safety, of fremanezumab, a monoclonal antibody targeting the calcitonin gene-related peptide (CGRP) pathway, for the preventive treatment of episodic migraine (EM) in pediatric patients aged 6 to 17 years.1
This multicenter, randomized, double-blind, placebo-controlled, parallel-group study enrolled 237 participants, who were randomized 1:1 to receive weight-based monthly subcutaneous injections of fremanezumab (120mg for participants <45kg; 225mg for ≥45kg) or matching placebo over a 12-week double-blind treatment period.1 Eligible participants had a documented diagnosis of migraine for at least six months and reported no more than 14 headache days per month at baseline.1 A total of 234 participants were included in the efficacy analysis (fremanezumab, n=123; placebo, n=111).1 Baseline characteristics were well balanced across both groups, with a mean age of 13.3 years in the fremanezumab group.1
The primary endpoint was the least-squares (LS) mean change from baseline in average monthly migraine days (MMD) during the 12-week treatment phase.1 Secondary endpoints included the LS mean change from baseline in monthly headache days of at least moderate severity (MHD) and the proportion of participants achieving a ≥50% reduction in MMD over 3 months.1 Additional secondary endpoints included the LS mean change from baseline in the average number of monthly days with acute migraine medication use over 3 months, as well as assessments using the Pediatric Migraine Disability Assessment (PedMIDAS) and the Pediatric Quality of Life Inventory (PedsQL).1 Predefined subgroup analyses were conducted by age group (6-11 years vs. 12-17 years) and sex.1
FINDINGS
| Primary endpoint: |
|
| Secondary endpoints: |
|
| Safety: |
|
References
- Hershey AD, et al. Efficacy and safety of fremanezumab for the preventive treatment of episodic migraine in children and adolescents: A phase 3, randomized, double-blind, placebo-controlled study. Presented at the American Academy of Neurology (AAN) Annual Meeting 2025; April 5-9, 2025.