Rapid resolution of recurrent GPP flares with spesolimab: Preliminary findings from the ongoing EFFISAYIL-REP study
15 Dec 2025
Share
STUDY DESIGN
Generalized pustular psoriasis (GPP) is a rare, potentially life-threatening autoinflammatory skin disease characterized by recurrent flares of sterile pustules and systemic inflammation.1 Spesolimab, a humanized monoclonal antibody targeting the interleukin-36 receptor, is approved as a 900mg intravenous (IV) formulation for the treatment of GPP flares based on results from the pivotal EFFISAYIL-1 study.1 The ongoing EFFISAYIL-REP study evaluates repeated IV spesolimab treatment in adults with recurrent GPP flares and explores the impact of immunogenicity on efficacy, safety, and pharmacokinetics.1
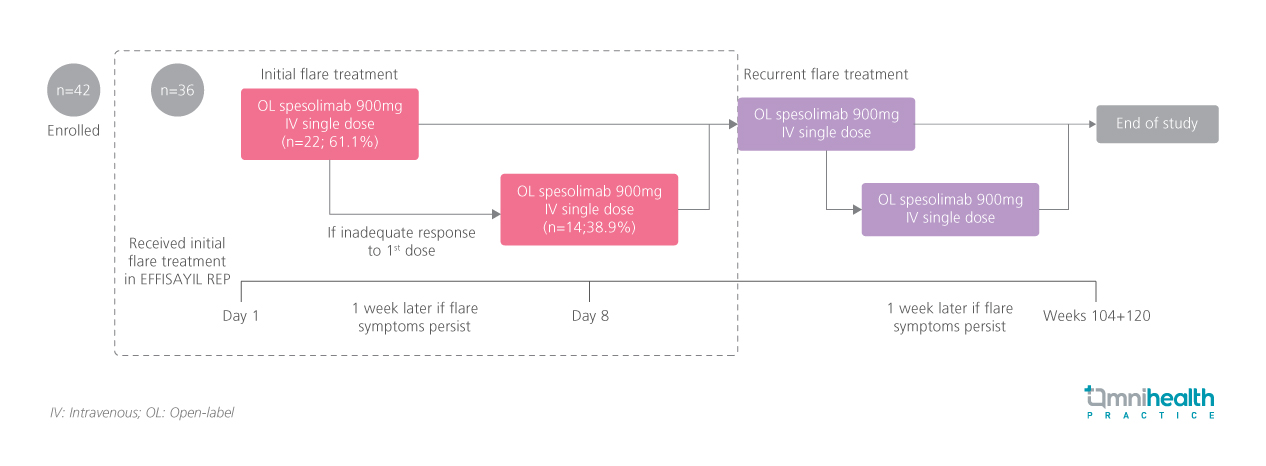
EFFISAYIL-REP is an open-label, multicenter, post-marketing study enrolling adults aged >18 years with a documented GPP diagnosis according to European Rare and Severe Psoriasis Expert Network (ERASPEN) criteria, and a high disease burden with frequent flares.1 Eligible patients on GPP treatment had a history of flares after dose reduction or discontinuation of concomitant GPP medication, or stopped treatment on the day of initial spesolimab administration (no washout period required).1 Those not on GPP treatment must have had ≥2 flares in the prior year.1 Concomitant medications could be continued if indicated for conditions other than GPP.1
The study included a flare-treatment period followed by a 16-week post-flare follow-up, for a total duration of approximately 104-120 weeks per patient.1 Flare treatment was initiated in patients with GPP flare, defined by a GPP Physician Global Assessment (GPPGA) pustulation subscore ≥2, with new or worsening pustules affecting ≥5% body surface area.1 Patients received a single 900mg IV dose of spesolimab on day 1 of the initial flare, with an optional second dose on day 8 if flare symptoms persisted or worsened.1
At the data cut-off, 42 patients were enrolled, with 36 receiving spesolimab for their first flares.1 The cohort had a slight female preponderance and was predominantly Asian, consistent with known GPP epidemiology.1 Mean age was 46.2 years, mean body mass index 27.3kg/m², and mean disease duration 8.8 years.1 Most patients presented with moderate pustulation (GPPGA score 2-3), and 16.7% had severe disease (score 4).1
The primary endpoint was a GPPGA pustulation subscore of 0 at week 1.1 The secondary endpoint was a GPPGA pustulation subscore of 0 or 1, with a ≥2-point reduction from baseline at week 1.1 Safety was monitored throughout the trial.1
STUDY DESIGN
| Primary endpoint: |
|
| Secondary endpoint: |
|
| Safety outcome: |
|
“IV spesolimab demonstrated rapid control of GPP flares during the first treatment episode”
Dr. Choon Siew Eng
Department of Dermatology,
Hospital Sultanah Aminah,
Johor Bahru, Malaysia
References
- Choon SE, et al. Spesolimab for the treatment of generalized pustular psoriasis flare: preliminary analysis of the EFFISAYIL REP study. Presented at the European Academy of Dermatology and Venereology (EADV) Congress 2025; September 17-20, 2025.